Hydrophobic Effect Described in One Word
Hydrophobic molecules are hydrophobic due to their non-polarity. The hydrophobic effect is caused by nonpolar molecules clumping together.

Hydrophobic Definition Examples Molecules Substances
At the macroscopic level the.

. For example by segregating to the oilwater interface amphiphilic molecules that possess both hydrophobic and hydrophilic groups can mitigate unfavorable oilwater interactions thereby. Tanford described how hydrophobic or oily groups prefer to get away from water while hydrophilic groups prefer to dissolve in the water. The word hydrophobic literally means water-fearing and it describes the segregation of water and nonpolar substances.
It results in the burial of the hydrophobic residues in the core of the protein. In this paper we summarize some of the theoretical efforts T ¼ T c and to cold denaturation at T u000230 u0005 C. And this is similar to a biochemical phenomenon called the HYDROPHOBIC EFFECT.
Posted by Unknown at 809 PM. The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The entropy of the solute is relatively minor for this reaction and the hydrophobic effect.
Water molecules order themselves into cages called clathrates around hydrophobic molecules that dont offer binding opportunities at the extreme things like fats and oils which dont readily mix with water kinda like those defensive walls formed around goals in soccer. It is exemplified by the fact that oil and water do not mix and was described well by G. Hydrophobic substances cannot be mixed with or dissolved in water.
Hartley in 1936. 1 2 The word hydrophobic literally means water-fearing and it describes the segregation of water and nonpolar substances which maximizes hydrogen bonding between molecules of water and minimizes the area of contact. Therefore the hydrophobic effect is the result of the entropy of water.
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. Because water is so good at forming hydrogen bonds with itself it is most hospitable to molecules or ions that least disrupt its Hbonding network. The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules.
The hydrophobic effect which is used to describe the aversion of oil for water or the affinity of oily objects for one another in water plays an important role in diverse disciplines. But if the droplet forms a sphere that barely touches the surface like drops of water on a hot griddle the contact angle is more than 90 degrees and. The name arises from the combination of water in Attic Greek hydro-and for fear phobos which describes the apparent repulsion between water and hydrocarbons.
Hydrophobic effect is an entropy-driven process that seeks to minimize the free energy of a system by minimizing the interface surface between hydrophobic molecules and water. Due to these contrasting scalings. The most efficient mechanism for reducing this waterhydrophobic interface is the aggregation of the hydrophobic surfaces with one another.
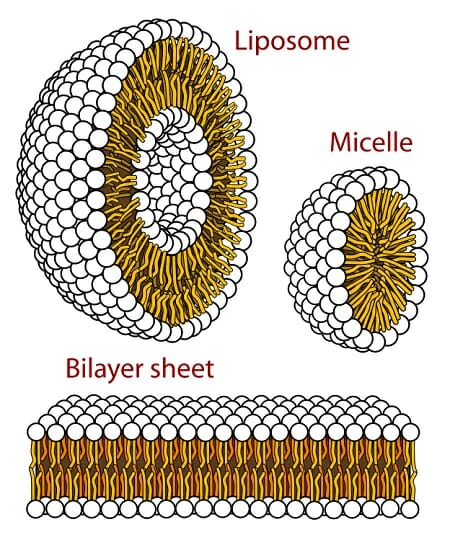
Large macromolecules can have hydrophobic sections which will fold the molecule so they can be close to each other away from water. The crossover from one regime to the other occurs when the oily species presents a surface in water extending over about 1 nm2. Watching oils float on the surface of water demonstrates that oil molecules are nonpolar they dont carry a charge or polarity and do not dissolve in water.
The hydrophobicity describes how much hydrophobic a molecule is. The hydrophobic effect describes the energetic preference of nonpolar molecular surfaces to interact with other nonpolar molecular surfaces and thereby to displace water molecules from the interacting surfaces. The hydrophobic effect is due to both enthalpic and entropic effects.
At intermediate to understand microscopically the hydrophobic effect and the role temperatures between T u000230 u0005 C and T c the folded configura-. The hydrophobic effect is considered to be the major driving force for the folding of globular proteins. The antipathy of the paraffin chain for water is however frequently misunderstood.
Many amino acids in proteins are hydrophobic helping the proteins obtain their complicated shapes. To investigate the mechanism why cholestasis may affect HSC activation we treated primary HSCs with one of the hydrophobic bile acids taurolithocholic acid for 24 hours. Hydrophobic effects12 Namely the free energy to solvate small hydrophobic molecules scales linearly with solute volume whereas that to solvate large hydrophobic species scales linearly with surface area.
If the droplet spreads wetting a large area of the surface then the contact angle is less than 90 degrees and that surface is considered hydrophilic or water-loving from the Greek words for water hydro and love philos. Hydrophobic molecules are molecules that do not dissolve in water. The hydrophobic effect is the property that non-polar molecules tend to form intermolecular aggregates in an aqueous medium and analogous intramolecular interactions.
The hydrophobic effect extends to organisms as. Hydrophobic is a term used in chemistry to describe the scientific phenomenon in which nonpolar substances are unable to dissolve in water. These hydrophobic molecules are called hydrophobes.
Therefore these molecules repel water molecules. Please take a moment and also remember that entropy S applies to the solvent water and not the solute oil. A droplet of water forms a spherical shape minimizing contact with the hydrophobic leaf.
Waterborne hydrophobic pollutants collect and magnify on the surface of plastic debris thus making plastic more deadly in the ocean than it would be on land. The word hydrophobic literally means water-fearing and it describes the segregation of water and nonpolar substances which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between. In other words hydrophobic molecules are nonpolar.
1 This simple activity demonstrates the concepts of the hydrophobic effect hydrogen bonding and can be used to explore detergents effect on the surface tension of water.

Hydrophobic Definition Examples Molecules Substances
Hydrophobic Definition And Examples Biology Dictionary

Hydrophobes Are Not Afraid A Psa On The Hydrophobic Effect The Bumbling Biochemist
Comments
Post a Comment